| Dec 08, 2025 |
New model explains stability and electronic behavior of boron nanostructures from hollow clusters to ultrathin 2D layers, linking them to atomic coordination.
(Nanowerk News) Boron, a chemical element next to carbon in the periodic table, is known for its unique ability to form complex bond networks. Unlike carbon, which typically bonds with two or three neighboring atoms, boron can share electrons among several atoms. This leads to a wide variety of nanostructures. These include boron fullerenes, which are hollow, cage-like molecules, and borophenes, ultra-thin metallic sheets of boron atoms arranged in triangular and hexagonal patterns.
|
|
Dr. Nevill Gonzalez Szwacki has developed a breakthrough model explaining the variety of boron nanostructures. The analysis presented in the article (2D Materials, “Coordination-driven design principles for boron fullerenes and borophenes: a predictive framework linking theory and experiment”) combines more than a dozen known boron nanostructures, including the experimentally observed B₄₀ and B₈₀ fullerenes.
|
|
Using first-principles quantum-mechanical calculations, the study shows that the structural, energetic, and electronic properties of these systems can be predicted by looking at the proportions of atoms with four, five, or six bonds.
|
 |
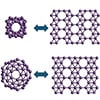
| Structural correspondence between boron fullerenes and 2D borophenes. The B₄₀ cage relates to the χ₃ borophene layer, while B₆₅ matches the β₁₂ layer. (Image: N. Gonzalez Szwacki, Faculty of Physics, University of Warsaw).
|
|
The results reveal clear links between finite and extended boron structures. The B₄₀ cage corresponds to the χ₃ borophene layer, while B₆₅, B₈₀, and B₉₂ connect with the β₁₂, α, and bt borophene sheets, respectively. These structural links suggest that new boron cages could be created by using known two-dimensional boron templates.
|
|
This coordination-based approach not only brings together previously separate structural families but also explains general trends: higher atomic coordination usually leads to greater stability of boron nanostructures, while their electronic properties depend more on geometry and how the orbitals are arranged. For instance, some cages like B₄₀ have large electronic gaps because of their compact and symmetrical shapes, while highly coordinated structures may be metallic or have smaller gaps. Therefore, the number of atomic connections serves as a unifying and predictive factor rather than a direct measure of electronic properties.
|
|
“The concept presented here serves as a guide for designing new boron nanostructures with specific magnetic, electronic, or mechanical features. It may also support future experiments using cluster-beam or surface-growth techniques,” emphasizes Dr. Nevill Gonzalez Szwacki.
|
|
The publication by the University of Warsaw researcher demonstrates that boron remains an exceptionally versatile platform for creating tunable nanoscale materials, bridging the molecular and two-dimensional worlds.
|