| Jun 05, 2025 |
Scientists developed a new fabrication technique to fine-tune a platinum catalyst on an atomic level.
(Nanowerk News) A research team led by the Department of Energy’s (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) designed and fabricated catalysts that can increase the speed of carbon monoxide oxidation by nine times. Carbon monoxide oxidation is an important reaction used in numerous chemical industry and environmental cleaning applications. The cutting-edge fabrication approach involved making precise, atomic-level changes in catalysts to create new, performance-boosting chemical properties.
|
|
“Our study provided deep insights into the chemical structure, reaction mechanisms, and performance of these advanced catalysts,” said Ji Su, a research scientist in Berkeley Lab’s Energy Technologies Area (ETA). “It sets the stage for a new era in superior catalyst design, with potential to dramatically improve the production efficiency of a wide range of chemical industry and environmental applications.”
|
|
The research was published in Science (“Formation of hydrided Pt-Ce-H sites in efficient, selective oxidation catalysts”). The collaborators included Oak Ridge National Laboratory and several other institutions.
|
 |
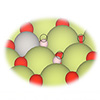
| The left molecular diagram shows how an individual platinum atom (gray) has replaced a cerium atom (green) on a cerium oxide surface. In the diagram at right, hydrogen molecules (white) applied to the surface split into hydrogen atoms that bond with cerium. This atomically tailored catalyst demonstrated impressive performance. (Image: Berkeley Lab)
|
A Workhorse in the Chemical Industry
|
|
Catalysts are materials that increase the speed of chemical reactions. The chemical industry relies heavily on catalysts to make production more cost-effective and higher quality, with about 95% of fuel and chemical products using one or more catalysts in their manufacturing process. Cars use catalysts to oxidize harmful emissions in exhaust, including unburned fuel and carbon monoxide.
|
|
Besides faster reaction speed, another desirable catalyst attribute is selectivity. This refers to the catalyst’s ability to activate more efficient reaction pathways, maximize output of desired products, and minimize waste products.
|
|
Traditionally, catalysts have been made by fabricating particles, or clusters of hundreds of atoms. In recent years, researchers have been investigating advanced fabrication techniques that involve manipulating individual metal atoms on a catalyst’s surface. The idea is to tailor chemical properties that enable faster, more selective catalytic reactions.
|
Like Mounting a Diamond and Building with Lego Bricks
|
|
The team developed a new treatment process that involves loading a single platinum atom onto a specific location on a cerium oxide surface, with the platinum replacing a cerium atom.
|
|
“The process is like mounting a diamond on a support structure on a ring,” said Su from ETA’s Energy Storage and Distributed Resource Division.
|
|
Next, in a process like building with Lego bricks, the researchers applied hydrogen molecules to the platinum-cerium structure. The hydrogen molecules split into atoms that bond with the cerium. For comparison, the team also made a control catalyst by randomly loading a platinum atom on a cerium oxide surface, without any hydrogen treatments.
|
Blazing Fast and More Selective
|
|
The team tested the catalysts’ performance in two reactions. The first was oxidation of carbon monoxide to yield carbon dioxide. The second was removal of hydrogen from propane to make propylene, which is an important raw material in plastics. The latter has emerged as a promising alternative to conventional propylene production.
|
|
The precisely tailored catalyst oxidized carbon monoxide nine times faster than the control catalyst. It was also 2.3 times more selective at converting propane to propylene.
|
|
Two important contributors were the Advanced Light Source and the Molecular Foundry, which are DOE Office of Science User Facilities located at Berkeley Lab. The researchers used the Molecular Foundry’s high-resolution imaging techniques to visualize platinum inserted into the surface. They also used the Molecular Foundry to conduct simulations to characterize the atomic structures and reaction pathways. Researchers at the Advanced Light Source applied a technique called ambient pressure X-ray photoelectron spectroscopy to determine the platinum’s charge (+2), helping the team better understand the platinum-cerium interactions.
|
|
In addition, the scientists used a neutron scattering technique at DOE’s Oak Ridge National Laboratory to characterize the bonding of cerium and hydrogen. Researchers used the synchrotron at Taiwan’s National Synchrotron Radiation Research Center to characterize the entire catalyst structure.
|
|
The team’s paper culminates a series of eight papers published by Su’s research group since 2019. Together, the studies paint a comprehensive picture of these innovative catalysts — their fabrication, structure, properties, chemical interactions, and effective performance.
|